LATEST NIH PUBLIC ACCESS POLICY NEWS: 2025
- 7/22/2025: Penn Libraries Federal Access Policies Guide
- 6/30/2025: ORS Reminder- Changes to NIH Public Access Policy go into effect July 1, 2025
- 5/9/2025: PSC's Information about the changes to the NIH PUBLIC ACCESS POLICY (see below)
|
Notice of Updated Effective Date for the new 2024 NIH Public Access Policy * Author Accepted Manuscript: The author’s final version that has been accepted for journal publication and includes all revisions resulting from the peer review process, including all associated tables, graphics, and supplemental material. * Official Date of Publication: The date on which the Final Published Article is first made available in final, edited form, whether in print or electronic (i.e., online) format. Supplemental Guidance to the 2024 NIH Public Access Policy:
MORE DETAILS BELOW |
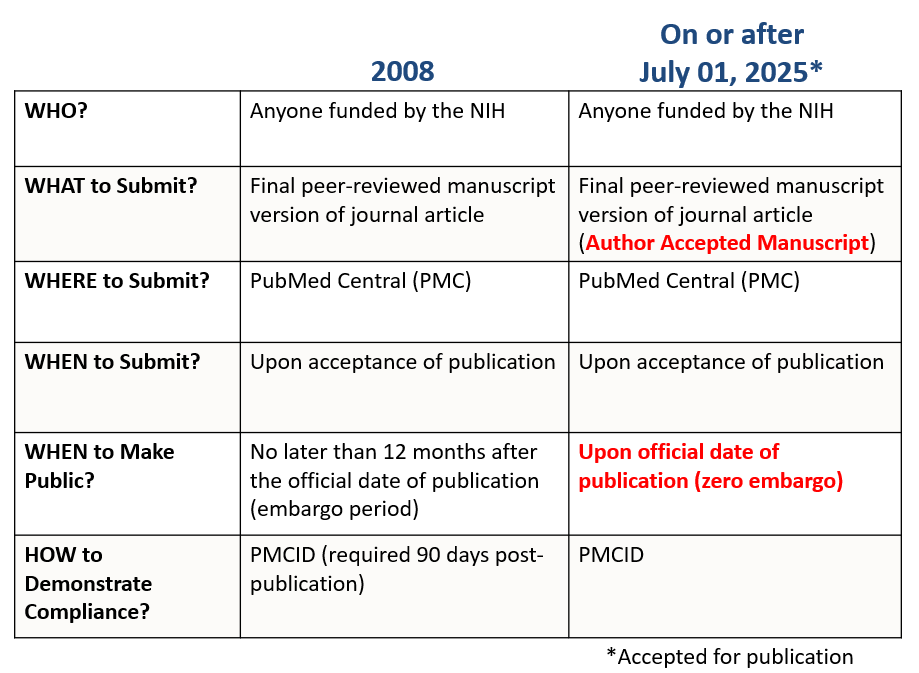
5/7/2025: The 2024 NIH Public Access Policy, which replaces the 2008 NIH Public Access Policy, will go into effect beginning on July 1, 2025 (April 30, 2025: NOT-OD-25-101).
WHAT DOES THIS MEAN FOR ME?
The process for submitting is the same as it has been under the 2008 NIH Public Access Policy, manuscripts will continue to be submitted via the NIHMS for inclusion in the full-text publicly accessible PubMed Central (PMC) database and can continue to be deposited via Methods, A, B, C, and D. NIH has defined some terms to make the policy clearer (see the set of definitions below).
The most significant changes from the previous policy is (1) the removal of the 12-month embargo period before manuscripts resulting from NIH funding must be made publicly available. The 2024 NIH Public Access Policy requires Author Accepted Manuscripts accepted for publication in a journal, on or after July 1, 2025, to be submitted to PubMed Central upon acceptance for publication, for public availability without embargo (2) upon the Official Date of Publication.
In the interim, authors should continue to follow the 2008 policy which allows for a 12 month embargo.
Best Practices & Requirements for Authors of peer-reviewed journal articles funded by NIH
- Discuss and communicate funding requirements with co-authors at the onset of your project.
- Ensure that journal publisher policies are in compliance with the NIH Public Policy mandate.
- Save all versions of your final peer-reviewed manuscript files, including tables, figures, supplemental files, and data products, in easy-to-access folders that are clearly labeled.
- Authors must acknowledge NIH funding in manuscripts. NIH provides the following sample language that may be included in the Submitted Manuscript and then, should it be accepted, the Author Accepted Manuscript, and NIH strongly encourages its use:
- “This manuscript is the result of funding in whole or in part by the National Institutes of Health (NIH). It is subject to the NIH Public Access Policy. Through acceptance of this federal funding, NIH has been given a right to make this manuscript publicly available in PubMed Central upon the Official Date of Publication, as defined by NIH.”
- Be aware of the NIH Grants Policy requirements, which state that each each publication, press release, or other document about research supported by an NIH grant must include: (1) An acknowledgment of NIH grant support such as: "Research reported in this [publication, release] was supported by [name of the Institute, Center, or other funding component] of the National Institutes of Health under grant number [specific NIH grant number in this format: R01GM012345]." And after the acknowledgement of funding, (2) A disclaimer that says: "The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Upon acceptance for publication, Author Accepted Manuscripts must be submitted to PubMed Central for public availability (without embargo beginning on July 1, 2025) upon the Official Date of Publication.
- Authors submitting Author Accepted Manuscripts to PubMed Central via the NIHMS must agree to a submission statement as part of the standard PubMed Central manuscript submission process (NOT-OD-25-049).
- Know that publisher fees for depositing the AAM to PubMed Central are not allowable, although publication costs for open access publishing of the final published article will still be allowed (within regulations), and authors will continue to be able to deposit Author Accepted Manuscripts for free via the NIHMS. Read more about Publication Costs (NOT-OD-25-048).
WHY WAS THERE A CHANGE?
"On December 17, 2024, NIH released its updated 2024 Public Access Policy. The Public Access Policy was informed by public feedback, including comments received in response to the NIH Draft Public Access Policy. The 2024 NIH Public Access Policy is in keeping with the White House OSTP memo “Ensuring Free, Immediate, and Equitable Access to Federally Funded Research”. The 2024 Public Access Policy is effective for manuscripts accepted for publication on or after ***July 1, 2025.*** Until then, NIH’s current Public Access Policy remains in effect." (Taken from this page https://osp.od.nih.gov/policies/public-access/)
Important Links & News about the Policy and Changes:
- Notice of Updated Effective Date for the 2024 NIH Public Access Policy
- NIH Director’s Statement on Accelerating Access to Research Results
- Under the Poliscope blog: Transforming Transparency Through Policy
- 2024 NIH Public Access Policy
- Supplemental Guidance: Publications Costs
- Supplemental Guidance: Government License Use and Rights
- NIH Director’s Statement on Release of 2024 NIH Public Access Policy
- Under the Poliscope Blog: Introducing the New NIH Public Access Policy
WHAT IS THE 2024 NIH PUBLIC ACCESS POLICY?
(Taken from this page: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-25-047.html)
Purpose
Increasing access to publications resulting from National Institutes of Health (NIH) funding offers many benefits to the scientific community and the public who funded the underlying research. When patients, families, and healthcare providers can access published findings resulting from NIH funding, they are able to better understand and address the most critical health concerns facing their communities. It also allows researchers, students, and members of the public in all communities to have equitable access to such content. This access can accelerate future research, lead to collaboration, and allow interested readers and patients to follow the latest advances more closely. Importantly, these goals also reflect NIH’s commitment to the responsible stewardship of the Nation’s investment in biomedical research by improving transparency and accessibility of taxpayer-funded research, an essential component of fostering trust in research.
To achieve these goals, the NIH Public Access Policy requires Author Accepted Manuscripts accepted for publication in a journal, on or after ***July 1, 2025,*** to be submitted to PubMed Central upon acceptance for publication, for public availability without embargo upon the Official Date of Publication.
Definitions
Author Accepted Manuscript: The author’s final version that has been accepted for journal publication and includes all revisions resulting from the peer review process, including all associated tables, graphics, and supplemental material.
Final Published Article: The journal's authoritative copy, including journal or publisher copyediting and stylistic edits, and formatting changes, even prior to the compilation of a volume or issue or the assignment of associated metadata.
Journal: A periodical publication that is either 1) included in the “journal” section of the National Library of Medicine (NLM) Catalog or 2) meets all of the following criteria:
- Requirements for ISSN assignment;
- Content is issued over time under a common title;
- Is a collection of articles by different authors; and
- Is intended to be published indefinitely.
Official Date of Publication: The date on which the Final Published Article is first made available in final, edited form, whether in print or electronic (i.e., online) format.
Scope and Effective Date
The NIH Public Access Policy applies to any Author Accepted Manuscript accepted for publication in a journal, on or after ***July 1, 2025,*** that is the result of funding by NIH in whole or in part through:
- A grant or cooperative agreement, including training grants,
- A contract,
- An Other Transaction,
- NIH intramural research, or
- The official work of an NIH employee.
The NIH Public Access Policy applies regardless of whether the NIH-funded principal investigator or project director is an author and regardless of whether non-NIH funds contributed to developing or writing the Author Accepted Manuscript. Upon the Effective Date, this Policy replaces the 2008 NIH Public Access Policy.
Requirements
The NIH Public Access Policy requires:
- Submission of an electronic version of the Author Accepted Manuscript to PubMed Central upon its acceptance for publication for public availability without embargo upon the Official Date of Publication;
- An acknowledgment in the Author Accepted Manuscript and Final Published Article that satisfies the requirements in the NIH Grants Policy Statement (GPS) regarding communicating and acknowledging federal funding (GPS 4.2.1 and GPS 8.2.1), as well as analogous requirements for acknowledging federal funding as incorporated into the terms of Other Transaction agreements and applicable contracts; and
- When an Author Accepted Manuscript is submitted to NIH1, agreeing to a standard license that mirrors that of the Government Use License at 2 CFR 200.315, or its successor regulation, explicitly granting NIH the right to make the Author Accepted Manuscript publicly available through PubMed Central without embargo upon the Official Date of Publication. 1(This happens typically through the NIH Manuscript Submission (NIHMS) System.)
Government Use License and Rights
- By accepting NIH funding, the recipient grants to NIH, as the funding agency, a royalty-free, nonexclusive, and irrevocable right to reproduce, publish, or otherwise use the work for federal purposes and to authorize others to do so, which includes making Author Accepted Manuscripts publicly available in PubMed Central upon the Official Date of Publication. A statement that conveys this point is incorporated into Notices of Award, the terms of Other Transaction agreements, and applicable contracts.
- NIH encourages authors to include a statement that indicates the Author Accepted Manuscript is subject to the NIH Public Access Policy and that this means that NIH, as the funding agency, has the right to make the Author Accepted Manuscript publicly available in PubMed Central upon the Official Date of Publication. NIH provides sample language in the Guidance on Government Use License and Rights that authors may choose to include in Author Accepted Manuscripts. Such a statement ensures transparency and ensures awareness that NIH has the right to make the Author Accepted Manuscript available in PubMed Central without embargo upon the Official Date of Publication.
- Authors are not expected to provide rights to NIH to the Final Published Article, and the rights that accrue to NIH upon the acceptance of funding are to the Author Accepted Manuscript. However, as noted in the section on Compliance and Enforcement, NIH will accept submission of the Final Published Article to PubMed Central from journals or publishers with formal agreements with NLM as compliant with the Policy when it may be made publicly available without embargo upon the Official Date of Publication.
NIH Funding of Publication Costs
Reasonable costs associated with publication that are allowable costs of the project budget may be requested as direct or indirect costs, as specified in the GPS 7.9 and as incorporated into the terms of Other Transaction agreements and applicable contracts (see the Guidance on Publication Costs for more information). Submission of Author Accepted Manuscripts to PubMed Central remains free for authors under the NIH Public Access Policy. If, during the course of the publication process, an author is asked to pay a fee for submission of the Author Accepted Manuscript to PubMed Central, such costs are not allowable.
Compliance and Enforcement
Regarding submission to PubMed Central, compliance with the Policy may be achieved through either:
- Submission of the electronic version of the Author Accepted Manuscript to PubMed Central upon its acceptance for publication, for public availability without embargo upon the Official Date of Publication, or
- Submission of the Final Published Article to PubMed Central from journals or publishers with formal agreements with NLM, upon the Official Date of Publication, for public availability without embargo.
Additional details on compliance and enforcement can be found below:
- Grants: Noncompliance with the NIH Public Access Policy may be considered by NIH regarding future funding decisions for the recipient institution (e.g., as authorized in the NIH GPS 8.5, Specific Award Conditions and Remedies for Noncompliance (Specific Award Conditions and Enforcement Actions)). Non-competing continuation grant awards are subject to a delay in award processing for noncompliance with the NIH Public Access Policy.
- Contracts: Compliance with and enforcement of the Policy will be consistent with the contract and the Federal Acquisition Regulations, as applicable.
- Other Transaction Agreements: Compliance with and enforcement of the Policy will be consistent with applicable NIH policies and the terms of the agreement.
- Intramural Research and the Official Work of NIH Employees: Compliance with and enforcement of the Policy will be consistent with applicable NIH policies and procedures.
Communicating and acknowledging federal funding enables a clear, public-facing indication of NIH funding in Author Accepted Manuscripts and Final Published Articles. Failure to include required acknowledgments may result in noncompliance with the NIH Public Access Policy, in addition to resulting in noncompliance with terms and conditions of funding regarding communicating and acknowledging federal funding.
FAQS
Q: Am I allowed to deposit my Author Accepted Manuscript in Penn's institutional repository as a working paper?
A: "NIH clarifies that the Policy does not prevent authors from depositing their Author Accepted Manuscripts into institutional repositories, as long as Author Accepted Manuscripts are also deposited in PubMed Central per the Policy." Also see your journal publisher policies to ensure there is no conflict.
---------------------------------------------------------------------------------------------------------------------------------------------------------
2024 News
Below are a few examples of how to Acknowledge NIH Grants for a variety of use cases. Minimally the grant number including the acronym NIH should be included.
The Population Studies Center (PSC) is supported financially by a Population Dynamics Research Infrastructure Program (PDRIP) award (P2C HD044964) from the National Institute of Health's (NIH), Eunice Shriver Kennedy National Institute of Child Health and Human Development's (NICHD), Population Dynamics Branch (PDB). The PDRIP program aims to advance the field of population dynamics research by increasing research impact, innovation, and productivity; developing junior scientists; and maximizing the efficiency of research support. The PDB supports research, data collection, and research training in demography, reproductive health, and population health. The PSC research themes are: New Dynamics of Population Diversity, Demography, Human Resources and Endowments, International Population Research, and New Directions in Population Research. Research Associates, Pilot Awardees, Population Center Working Paper authors, and other researchers who are supported by staff at the PSC and who conduct PDB-relevant research are being supported by the PSC's P2C grant (P2C HD044964) and should cite the grant in any peer-reviewed journal articles.
The Population Aging Research Center (PARC) is supported financially by award NIH P30 AG012836 from the Division of Behavioral And Social Research (BSR) of the National Institute of Aging (NIA) of the National Institute of Health's (NIH) via the Centers on the Demography and Economics of Aging program. PARC is one of twelve general Centers on the Demography and Economics (D&E) Aging and three Centers on the D&E of Alzheimer’s Disease/Alzheimer’s Disease and Related Dementias (AD/ADRD), which has an overarching coordinating center. The NIA's BSR also supports the program through the Research Centers Collaborative Network (RCCN) coordinating center that organizes efforts across each of the six types of NIA centers/programs. PARC's research themes are: Health Care and Long-Term Care in Older Adults, Cognition and Alzheimer's Disease and Related Dementia (ADRD), Health Disparities in Aging, Early Life-Conditions and Older Adult Health, Behavior and Well-Being, and Global Aging and Health. Additionally, PARC has five research networks: Global Family Change (GFC) Network, Latin American Network on Aging (LANA), Network on Aging in Sub-Saharan Africa (NASSA), Partnership to Improve Care and Translate Evidence for Seniors (PICANTES), and Network on Migration Advantage (NeMA). The PARC PIs, Pilot Awardees, Population Center Working Paper authors, and other researchers who are directly funded by PARC or who are significantly supported by staff at the PSC and who conduct research that falls within a research theme or a supported research network should cite the PARC grant NIH P30 AG012836 in peer-reviewed journal articles.
Citing a research project grant along with the PSC & PARC grants (used in cases where the PI or an author was supported additionally by the centers, an example of which includes the publication of a working paper, research brief, or website):
-- Funding for the XX Project is provided through the National Science Foundation (NSF Grant #######, PI: ______). This work was additionally supported by the Eunice Shriver Kennedy National Institute of Child Health and Human Development Population Research Infrastructure Program (NIH P2C HD044964, PI: Parrado) & the National Institute on Aging (NIH P30 AG012836, PIs: Kohler & Coe).
Examples you could use when you are the recipient of a Pilot Grant, please see your award letter for are a few other examples and for the relevant grant(s):
-- LONG VERSION: This paper was supported by a pilot received from the Population Studies Center at the University of Pennsylvania which is funded by the Eunice Shriver Kennedy National Institute of Child Health and Human Development Population Research Infrastructure Program (NIH P2C HD044964, PI: Parrado). The content is solely the responsibility of the authors and does not necessarily represent the official views of the University of Pennsylvania or National Institutes of Health.
-- SHORT VERSION: This paper was supported by the NIH P2C HD044964.
-- MEDIUM LENGTH: Research reported in this publication was supported by the NICHD of the National Institutes of Health (NIH) under award number P2C HD044964.
-- MEDIUM LENGTH: This paper received pilot funding from the Population Aging Research Center at the University of Pennsylvania, which is supported by the National Institute on Aging (NIH P30 AG012836).
Two examples for use when you are an NICHD Trainee, where the T32 is the sole source of funding for at least one of the authors:
-- This research received support from the Population Research Training Grant (NIH T32 HD007242, PI: Guillot) awarded to the Population Studies Center at the University of Pennsylvania by the National Institutes of Health’s (NIH)’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
-- Trainee Name acknowledges receipt of NIH funding, T32 HD007242 (PI: Guillot), while working on this research.
---------------------------------------------------------------------------------------------------------------------------------------------------------
2013 News
12/3/2013: Student NIH Public Access Policy Citation Guide
Sample acknowledgment text:
-- This research received support from the Population Research Training Grant (NIH T32 HD007242) awarded to the Population Studies Center at the University of Pennsylvania by the National Institutes of Health’s (NIH)’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
-- This research received support from the Population Research Training grant (NIH T32 HD007242) from the National Institutes of Health’s (NIH)’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and from the Demography of Aging grant (NIH T32 AG000177) from the National Institutes of Health’s (NIH)’s National Institute on Aging awarded to the Population Studies Center at the University of Pennsylvania.
-- We are grateful to the Population Studies Center at the University of Pennsylvania for general support (NIH grant number: R24 HD044964).
12/3/2013: Research Associate NIH Public Access Policy Citation Guide
Sample acknowledgment text:
-- This publication was made possible by funding from the Population Research Infrastructure Program of the National Institutes of Health’s (NIH)’s Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the Population Studies Center at the University of Pennsylvania, NIH grant number: R24 HD044964.
-- This research received support from the Population Research Infrastructure Program grant (NIH R24 HD044964) of the National Institutes of Health’s (NIH)’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and from the Centers on the Demography of Aging grant (NIH P30 AG012836) of the National Institutes of Health’s (NIH’s) National Institute on Aging awarded to the University of Pennsylvania.
-- We are grateful to the Population Studies Center at the University of Pennsylvania for general support (NIH grant number: R24 HD044964).
10/15/2013: PSC's Guide to the NIH Public Access Policy
10/15/2013: Click to view the PSC Presentation about the NIH Public Access Policy
7/10/2013: Penn Libraries NIH Public Access Policy Guide
7/1/2013: For non-competing continuation grant awards with a start date of July 1, 2013 or beyond: 1) NIH will delay processing of an award if publications arising from it are not in compliance with the NIH Public Access Policy. 2) Investigators will need to use My NCBI to enter papers onto progress reports. Papers can be associated electronically using the RPPR, or included in the PHS 2590 using the My NCBI generated PDF report. For an overview of policy changes, see this video, excerpted from our January 2013 WEBINAR.
---------------------------------------------------------------------------------------------------------------------------------------------------------
2009 News
Brief Introduction to the NIH Public Access Policy
Introduction to the Policy
The NIH Public Access Policy ensures that the public has access to the published results of NIH funded research. As of April 7, 2008, it requires scientists to submit final peer-reviewed journal manuscripts that arise from NIH funds to the digital archive PubMed Central upon acceptance for publication. To help advance science and improve human health, the Policy requires that these papers are accessible to the public on PubMed Central no later than 12 months after publication. The NIH Public Access Policy implements law Division G, Title II, Section 218 of PL 110-161. Penn's Office of Research Services prepared a "Brief Summary of the Revised NIH Public Access Policy."PSC Authors and Principal Investigators please read the NIH Notice Number: NOT-OD-08-033 Revised Policy on Enhancing Public Access to Archived Publications Resulting from NIH-Funded Research. Also read the "Letter to the University Research Community," from Penn's Vice Provost for Research, Steven J. Fluharty from April 8, 2009.
You can see an additional list of helpful links on our NIH Public Access Policy Resources Page, or read our special NIH edition of the PSC Information Services Newsletter.
Who does the policy apply to?
All authors (faculty, staff, students, and other researchers) who have received direct funding from an NIH grant. Direct funding generally includes sub-awards because they are associated with a particular award. An example of a sub-award at the PSC is a pilot/TRIO award. Receipt of general support for research from the Population Studies Center or the Population Aging Research Center (e.g., computing or library services, consultation with respect to human subjects) under core NIH awards such as the NICHD R24 or NIA P30 grants does not constitute "direct funding.
Students: "Being supported as a student on a T32 or other training award does constitute direct support. NIH requires us to track T32 trainees and their publications for 10 years and to accurately report the PMCIDs for those works that are published that are affected by the Policy (e.g. it is a peer-reviewed journal article, supported by NIH, and was published on or after April 7, 2008). The NIH also requires us to remind T32 recipients about compliance for those works that might be affected and to ensure that any peer-reviewed journal articles (published after April 7, 2008) resulting from T32 support are made available in PubMed Central.
To what papers does the policy apply?
The Policy applies to any manuscript that:
- Is peer-reviewed;
- And, is accepted for publication in a journal on or after April 7, 2008;
- And, arises from:
- Any direct funding from an NIH grant or cooperative agreement active in Fiscal Year 2008, or;
- Any direct funding from an NIH contract signed on or after April 7, 2008, or;
- Any direct funding from the NIH Intramural Program, or;
- An NIH employee.
Authors may submit final peer-reviewed manuscripts accepted before April 7, 2008 that arise from NIH funds, if they have appropriate copyright permission. Direct funding means costs that can be specifically identified with a particular project or activity.
What are the responsibilities of an NIH PI?
Principal Investigators and their Institutions are responsible for ensuring all terms and conditions of awards are met and that authors are aware of and comply with the NIH Public Access Policy. This includes ensuring that the submission of final peer-reviewed manuscripts that arise directly from their awards, even if they are not an author or co-author of the paper are deposited into the NIH Manuscript Submission System (NIHMS). In addition, PI's must cite the PMCID or NIHMSID numbers for articles arising from their awards in subsequent grant proposals (e.g. in biosketches, reference lists, etc.).
How to Comply With the Policy (a 3 step process)
Step 1. Address Copyright. Before you sign a publication agreement or similar copyright transfer agreement, make sure that the agreement allows the paper to be submitted to NIH in accordance with the NIH Public Access Policy. You can use Penn's "Suggested Cover letter for Author Journal Submission." If you have already completed the agreements and did not secure copyright, but are required to under law by the NIH Public Access Policy you must contact the publisher immediately to find out what their policy is on this issue to ensure that the final peer-reviewed manuscript is made available in PubMed Central no later than 12 months after publication. You can use this post acceptance letter to ask for an ammendment to your copyright agreement (it is in Word and is entirely modifiable- you should change all grey areas): Publishers_letter_post-acceptance.
Step 2. Submit the paper to NIH. This can be done in a number of ways. These methods vary in the version of the paper submitted, and the actions undertaken by the author and publisher.
A. Publish in a journal that deposits all final published articles in PubMed Central (PMC) without author involvement. For a list of the journals that submit all NIH Funded published articles to PubMed Central without author involvement go to: http://publicaccess.nih.gov/submit_process_journals.htm
B. Make arrangements to have the publisher deposit a specific final published article in PubMed Central.
C. Deposit the final peer-reviewed manuscript in PubMed Central yourself via the NIH Manuscript Submission System (NIHMS).
D. Complete the submission process for a final peer-reviewed manuscript that the publisher has deposited in the NIH Manuscript Submission System (NIHMS).Step 3. Cite. As of May 25, 2008, when citing a paper in NIH applications, proposals, and progress reports that falls under the Policy, and was authored or co-authored by you or arose from your NIH award, you must include the PubMed Central reference number (PMCID). This policy includes applications submitted to the NIH for the May 25, 2008 due date and subsequent due dates. Intramural researchers must ensure a PubMed Central reference number (PMCID) is included in the Institute’s Annual Report for any publication they have authored or co-authored.
Five things you should have available when you submit a manuscript
1. Your eRA Commons username and password. An eRA Commons account is for NIH Extramural principal investigators, grantees or applicants. Alternatively you may have an NIH Login (for Intramural NIH scientists and staff), an HHMI Login (for HHMI-funded investigators), or an MyNCBI (for third party submitters). If you don’t have one of these logins you can sign up for one on the NIHMS page.
2. Journal Name
3. Manuscript Name
4. Grant Number(s) or at minimum the PI’s name – you can look up the PI’s grant numbers
5. Manuscript File(s)How do I submit manuscripts via NIHMS? (from: http://www.nihms.nih.gov/faq.html#q4)
Step 1: Log in: Users can log in to NIHMS using their eRA Commons login or their NIH login. Third party submissions may be made by My NCBI account holders. Publisher login accounts are available for publishers interested in submitting manuscripts on behalf of authors to NIHMS.Step 2: Designate support: Submitters select the journal name, enter the manuscript title, and select all appropriate (NIH and/or HHMI) funding mechanisms that supported the manuscript.
Step 3: Upload manuscript: After providing basic information about the manuscript and contact information, users can upload their manuscript file(s). The system will generate a receipt of the uploaded files in PDF format. The PDF Receipt summarizes the information entered into the system, and merges the manuscript's files into one viewable document. Submitters confirm that the manuscript and any additional supporting documents have been successfully received by NIHMS. The NIHMSID is assigned at this time.
Step 4: Submission agreement and processing: An email will be sent to the Principal Investigator (PI) or corresponding author to approve the PDF and indicate the release date when the manuscript will be made publicly available on PMC. Upon approval by the submitter and the PI/author, the manuscript will be converted into XML - the standardized digital format used by PubMed Central.
Step 5: Approval of the converted manuscript (web version):
After the conversion process, the PI/author will review a version of the manuscript as it will appear in PubMed Central. At this time corrections may be requested, if necessary. After final approval, the article will be publicly accessible through PubMed Central after the time-delay specified by the PI/author.Sample Citations
- Chao, L.-W., J.A. Pagan, and B.J. Soldo. 2008. "End-of-Life Medical Treatment Choices: Do Survival Chances and Out-of-Pocket Costs Matter?" Medical Decision Making 28(4):511-523. NIHMSID: NIHMS69550.
- Grajeda, R., J.R. Behrman, R. Flores, J.A. Maluccio, R. Martorell, and A.D. Stein. 2005. "The Human Capital Study 2002-04: tracking, data collection, coverage, and attrition." Food and Nutrition Bulletin 26(2 Suppl 1):S15-24. PMCID: PMC1978414.
- Cerrato, A., et al. 2006. “Genetic interactions between Drosophila melanogaster menin and Jun/Fos.” Developmental Biology 298(1):59-70. PMCID: PMC Journal - In Process
Funding for the GFC Project is provided through the National Science Foundation (Grant 1729185, PIs: Kohler & Furstenberg). This work was additionally supported by the Eunice Shriver Kennedy National Institute of Child Health and Human Development Population Research Infrastructure Program (NIH P2C HD044964. PI: Parrado) & the National Institute on Aging (NIH P30 AG012836, PIs: Kohler & Coe).